
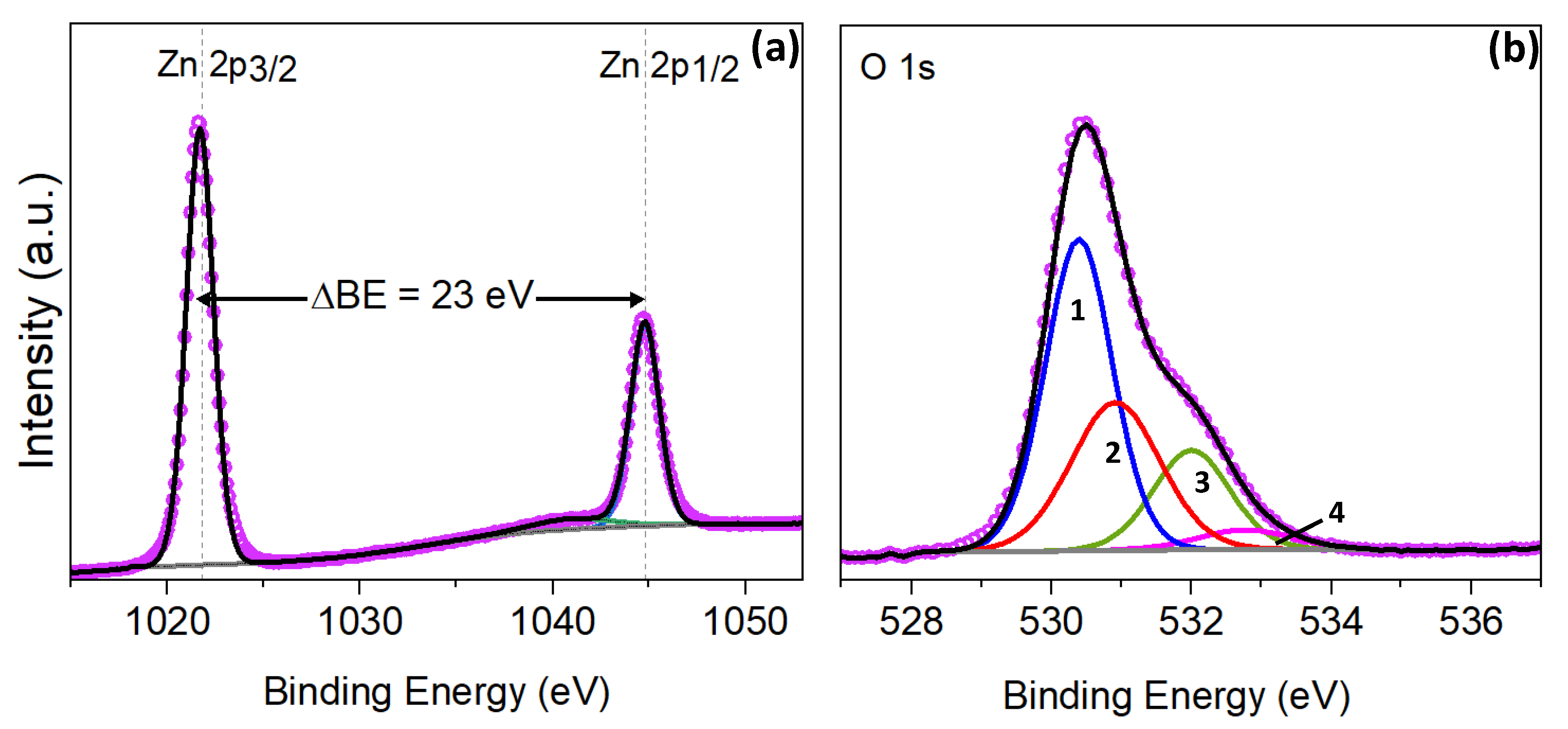
It is known that the luminescence, optical, and electrical properties of ZnO device structures are largely determined by the surface states and effects that take place on the surface of nano- and microcrystallites of ZnO films, nanorod arrays and nanoparticles. Due to its unique electrical, optical, luminescent and catalytic properties, ZnO is a promising material for various applications, including chemical sensors, transparent field effect transistors, transparent conducting oxide, atomic force microscopy cantilever, nanopiezoelectric devices, UV detectors, etc. Zinc oxide is an n-type conductivity semiconductor with a wide band gap of 3.37 eV, strong luminescence at room temperature, high conductivity and optical transparency. The annealing transforms the first type of site into the second one, and the subsequent short-term plasma treatment in hydrogen results in steady passivation, where the degradation of characteristics is practically reduced to zero. The XPS components of these sites include the Zn2p 3/2 peak at 1022.2 ± 0.2 eV and Zn2p 1/2 peak at 1045.2 ± 0.2 eV, with a part of the XPS O1s peak at 531.5 ± 0.3 eV. The second type of surface sites has a stable structure after hydrogen passivation and corresponds to HO–ZnO sites. The first type of surface sites is rapidly depassivated after hydrogen passivation and the aging effect takes place due to these centers. It was found that ZnO samples demonstrate different behavior of the degradation process, which corresponds to at least two different types of adsorbing surface sites for O 2, where O 2 adsorption is of a different nature. In this paper, the aging effect in ZnO films and nanorod arrays was studied. The practical use of nano- and polycrystalline ZnO devices faces a serious problem of instability of electrical and luminescent characteristics, due to the adsorption of oxygen by the surface during aging.
Zinc oxide is a promising multifunctional material.


 0 kommentar(er)
0 kommentar(er)
